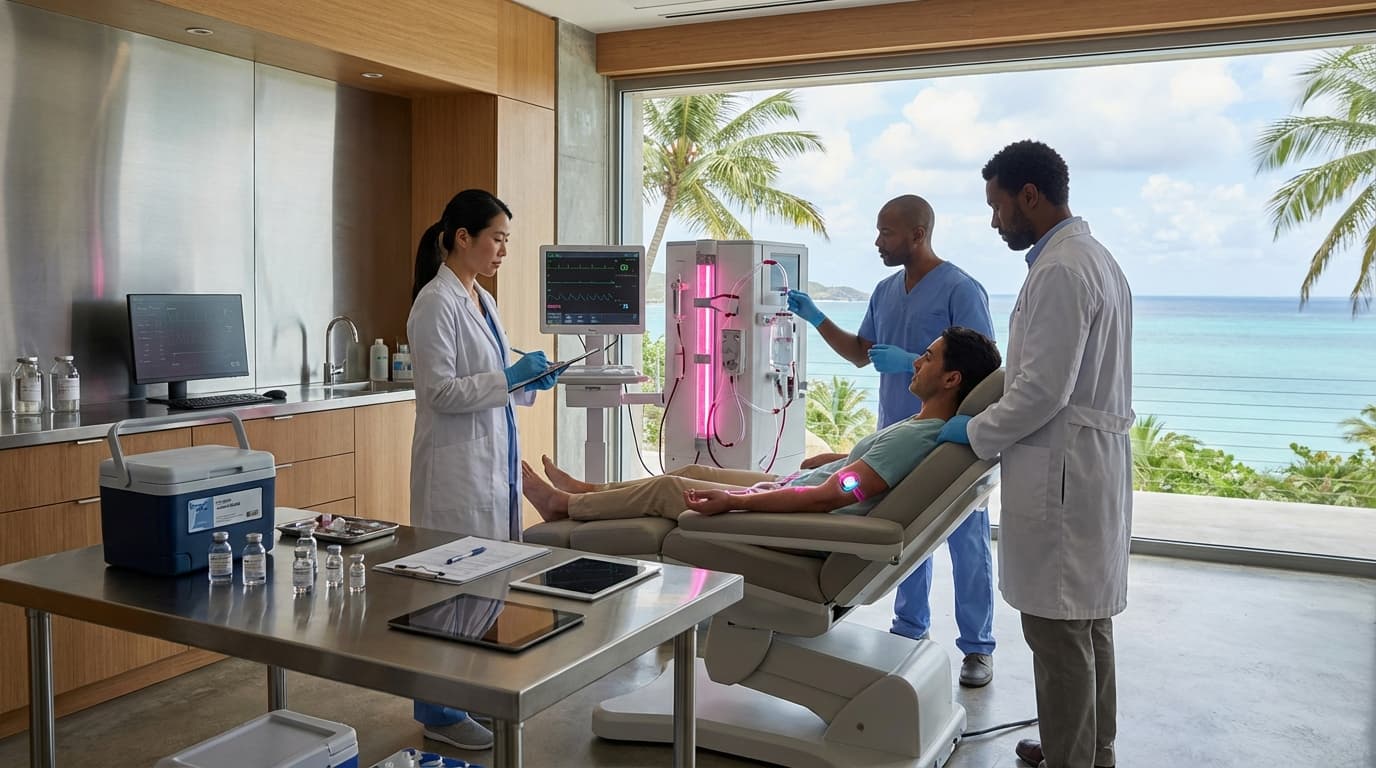
Chinese stem cell tourism refers to a phenomenon where clinics in China offer experimental regenerative medicine therapies including mesenchymal stem cell (MSC) infusions, exosome therapy, and cosmetic stem cell procedures to international clients, operating with relatively lax regulatory oversight compared to Western countries. These clinics market directly to global clients seeking treatments that may not be available or approved in their home countries, creating a regulatory arbitrage situation where patients travel to access therapies with uncertain safety and efficacy. This phenomenon highlights risks associated with regulatory differences between countries and informs bioethics policy discussions worldwide about how to balance patient access with safety and evidence-based medicine.
This innovation (or more accurately, this phenomenon) addresses demand for regenerative medicine therapies, where patients seek treatments that may not be available in their home countries due to regulatory restrictions. However, it raises significant concerns about patient safety, unproven treatments, and regulatory oversight. The situation demonstrates challenges in global regulation of medical treatments.
The technology raises important questions about medical tourism, regulatory oversight, and patient safety, where differences in regulation between countries create opportunities for potentially risky treatments. As regenerative medicine advances, these issues become increasingly important. However, ensuring patient safety, preventing exploitation, and maintaining evidence-based standards remain critical challenges. The phenomenon represents a complex issue in global healthcare, where patient autonomy, safety, and regulatory oversight must be balanced. Addressing these challenges requires international cooperation and careful consideration of how to protect patients while enabling legitimate medical innovation.